We are at the end of another hot, dry summer. Redwoods, pines and other salt sensitive trees have taken another beating.
What we all need is a good wet winter. But if your trees have a significant buildup of salt, all the water in the world will not help. When salt buildup is severe, water remains unavailable to the roots and trees slowly die of thirst.
Redwoods and pine trees are especially sensitive to salts. Salts weaken the trees and can lead to dieback or death. These trees are sensitive to salts in general (which alone can cause a certain amount of damage), but the main culprits that cause most of the damage and death are carbonates and sodium.
Let’s look at how this chain reaction occurs.
The Chain Reaction that Ends in Tree Death
Bicarbonate (HCO3) is a salt that's common in irrigation water. When bicarbonate gets into our soils and dries out, it releases the hydrogen (H), replaces it with calcium (Ca), and becomes calcium carbonate (CaCO3) - an insoluble mineral crystal that is known to plug pore spaces. Pore spaces are critical to tree health because they allow both air and water to reach the root system.
The formation of calcium carbonate is the first step in the declining health of these trees.
The next step is the inability to leach. Because the calcium carbonate has plugged the pore spaces, water can’t move through the soil profile as it needs to. Leaching or flushing becomes harder and harder. With the lack of cleansing through flushing, sodium begins to build up and soon the trees begin to pull that sodium up. Sodium is quite mobile and it moves to the outside edges of the leaves, resulting in sodium tip burn or firing around the edges of the leaves.
As the sodium buildup continues, you'll see stems, twigs, branches and limbs begin to die back. If you ignore the signs and allow the buildup to continue, it could very well lead to the death of the tree.
So, what do we do? What can be done?
Revitalizing Salt Damaged Trees with a Drench Application
One of the best ways to revitalize salt damaged trees is with a drench application. Drenching helps with salt issues and releases the sodium from the soil particles, unblocking the soil pores. This allows the water and salts to flush and moves the salt away from the root system.
To create 100 gallons of drenching solution, you'll need:
- ½ gallon of Blast Sprayable (for the calcium carbonate)
- ½ gallon of Caltrisal ST (for the sodium)
- A liquid calcium product of your choice (for sodium exchange)
- ½ gallon of Sixteen90 (to move the water deeper)
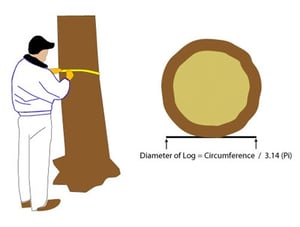 The application rate is 10 gallons of solution per 12” of diameter at breast height (4.5' above the ground). If you don't have a diameter measuring tool, measure the circumference and divide by 3.14. So if you're working with a redwood or pine tree with a 24" diameter, you'll need 20 gallons of solution.
The application rate is 10 gallons of solution per 12” of diameter at breast height (4.5' above the ground). If you don't have a diameter measuring tool, measure the circumference and divide by 3.14. So if you're working with a redwood or pine tree with a 24" diameter, you'll need 20 gallons of solution.
This application should be made within the dripline of the tree branches. And it should be made in November so the treatment is down before the rainy season gets started. That way you can take advantage of every drop of rain possible.
For more information about the program or the products, please contact your local Horizon Store or Horizon BDR.
About the Author:
Ken Mauser is a Consulting Agronomist who has served as Aquatrols' Territory Manager in the Western United States for more than 20 years.

 Much has been written about how to conserve water in a turf and landscape situation. And well it should. Water is our most precious resource. We can’t live without it, and we need to do all that we can to conserve it and preserve it, quantity and quality wise.
Much has been written about how to conserve water in a turf and landscape situation. And well it should. Water is our most precious resource. We can’t live without it, and we need to do all that we can to conserve it and preserve it, quantity and quality wise.